Are Fresh Water Boundaries the Future of Energy Harvesting?
The osmotic pressure difference between fresh water and seawater boundaries is being utilized to generate energy. This method of creating energy is known as "blue energy".
Researchers have developed a nano-sized membrane that can capture energy from osmosis. Is this the dawn of "blue energy"?
Capturing Energy in Estuaries
There are quite a number of renewable energy sources as of today. The "green" movement has seen the rise of energy harvesting from sources like solar, geothermal, and hydro-power.
However, some days, the sun doesn't shine and the wind doesn't blow, causing a limit on how much power some of these sources can produce. So what harm could one more source of clean energy do?
Researchers at the Ècole Polytechnique Fédérale de Lausanne (EPFL) might have found a way around the limitations set on some renewable sources of energy.
Their new energy source? The boundaries between seawater and fresh water.

An example of a seawater and fresh water boundary in Alaska. Image courtesy of Jane and Phillip Boger.
On July 13th, 2016, the research team's discoveries were published in the journal, Nature. The team developed a unique way to produce a large amount of energy at any fresh-seawater boundary.
The energy is produced through osmosis, which occurs when water with a high salt content comes in contact with fresh water through a permeable membrane. When seawater enters fresh water, salt ions are passed through, which have an electrical charge. The ions continue to pass through until the salinity of each body of water is at equilibrium.
So what does all of this have to do with renewable energy? If one can create a layer of material and place it at the crossing point the bodies of water, the electrical energy passed through the salt ions could potentially be harnessed for use.
This is exactly what the researchers at EPFL's Laboratory of Nanoscale Biology have done. They created a membrane that is comprised of only three atoms—yes, only three. This semipermeable membrane, which is made of MoS2 (molybdenum disulfide), is used to separate the two fluids with different salt concentrations.
Designing a Nano-Scale Membrane
Knowledge about the process of ions transferring between fresh and seawater isn't new, but rather it is EPFL's membrane that is cutting-edge. By producing such a small membrane, a greater current can be obtained due to the decrease of resistivity.
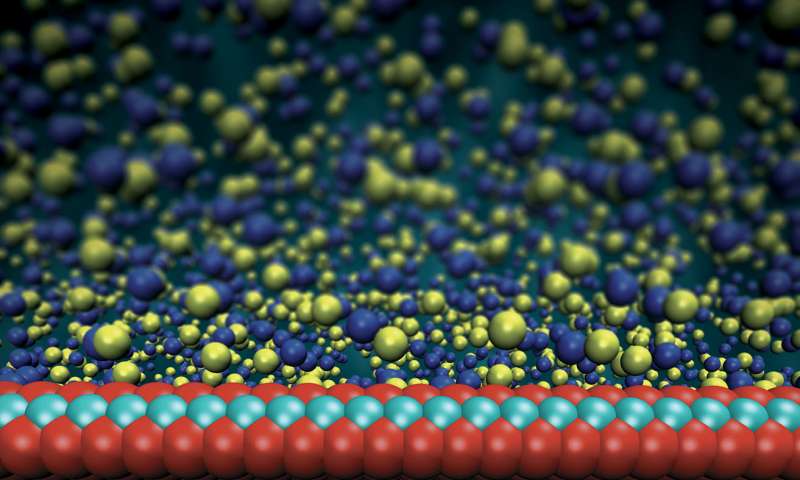
Rendering of the membrane and water molecules. Image courtesy of Steven Duensing, National Center for Supercomputing Applications.
Molybdenum disulfide is rather cheap as a material, which is a huge plus if the membrane were to be scaled across hundreds of miles. The membrane is composed of a vast amount of pores (of the nanometer scale) which allows the salt ions to pass through the membrane and harnesses the ions charge to generate electricity. Essentially, the ions pass through the nanopores and their electrons are transferred into electrodes.
Fortunately, the researchers' choice of material in the membrane allows for the positively charged ions to pass through whilst repelling most of the negatively charged ions. This creates a potential difference and effectively a voltage difference between the two liquids as the positive ions and negative ions build up. It is this voltage that allows the generated current to flow readily.
Jiandong Feng, the lead author of the research, has expressed that one of the challenges his team faced was determining the size of the openings in the membrane that the molecules can pass through. These openings are called nanopores.
According to Feng, making the nanopores too large would allow negative ions to pass through. Those electrons carried through the membrane would essentially be wasted and the voltage gathered by the membrane would be low as a result. On the other hand, if the nanopores are too small, potentially fewer ions could pass through the membrane at all leaving the current ultimately too weak.
Potential for Renewable Energy
The use of such a small membrane could lead to an astonishing amount of energy harnessed from a simple chemical reaction.
According to the researchers' analysis and calculations, from a 1 m2 MoS2 membrane with only 30% of its surface coated by the nanopores, 1 MW of electricity can be produced. That's enough to provide power to 50,000 energy saving light bulbs!
The molybdenum disulfide compound is naturally found and can also be created by a process known as chemical vapor deposition. With this material being so readily available, the membrane can be scaled for a larger amount of energy production. However, scaling is still a challenge because creating uniform pores throughout the membrane is proving difficult.
Nevertheless, this is a problem that can be eventually solved. The research team ran a nanotransistor from the current that was generated through a single nanopore and found that it demonstrated itself to be a self-powered nanosystem.
Nano-Membranes in Creating Potable Water
EPFL is not the only one researching this topic. They have been working in tandem with a group of researchers led by Professor Narayana Aluru at the University of Illinois at Urbana–Champaign. The US-based team has aided the EPFL team with molecular dynamic simulations to help predict molecule behavior and better design the membranes and nanopores.
Aluru's interest in working with molybdenum disulfide membranes, however, does not focus on the potential for harvestable energy. Instead, his team has been looking at these membranes as a way to convert salt water into potable fresh water.
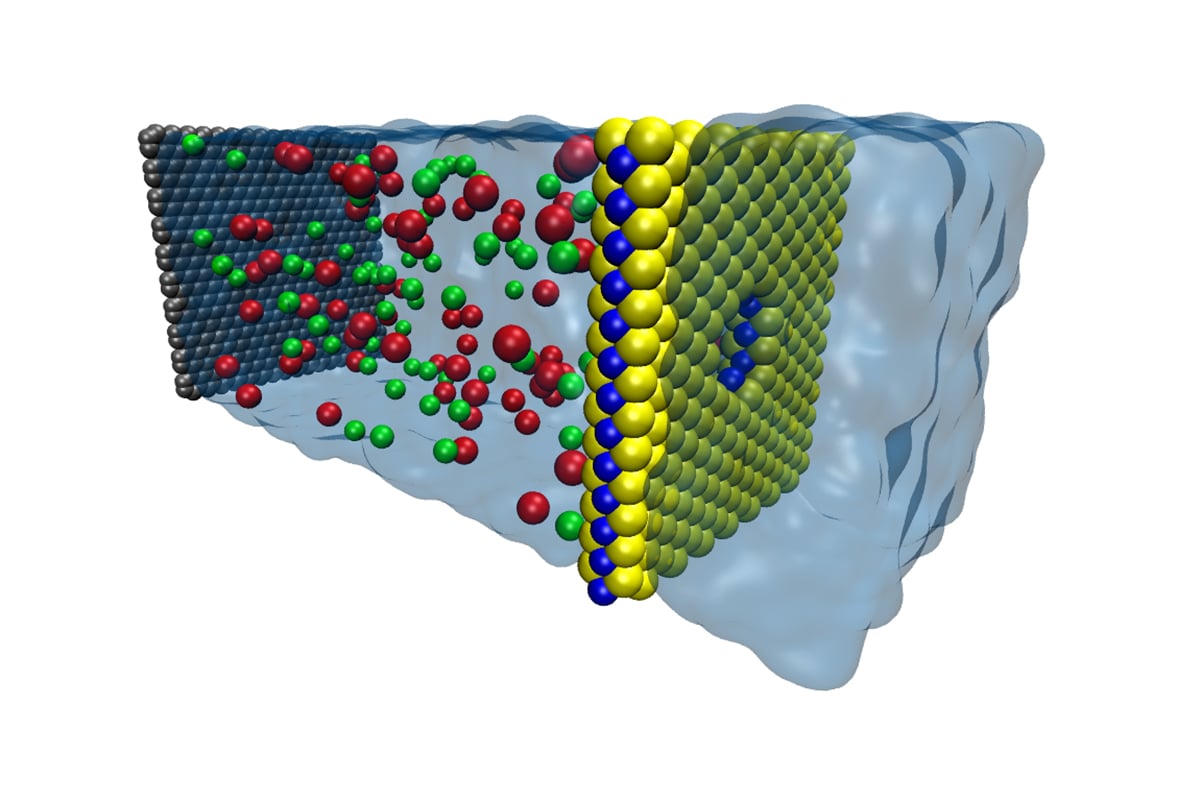
A rendering of the molybdenum sulfide membrane (with nanopore) being used as a filter to create fresh water. Image courtesy of the University of Illinois.
In short, this membrane technology could leverage the basic process of osmosis to not only produce sustainable energy but also provide drinkable water.
Blue energy is a growing trend which could gain massive traction in the upcoming years as the technology advances.
Once their engineering model and calculations become more efficient, we could see a future where blue energy is widely available. The simplistic process known as osmosis could play a tremendous part in generating renewable energy and beyond.








Making electricity from fresh water and seawater is not a new technology, in Holland since 2014 there is already a small powerplant to do exactly that.
http://www.dutchwatersector.com/news-events/news/12388-dutch-king-opens-world-s-first-red-power-plant-driven-on-fresh-salt-water-mixing.html
Jimmy in that plant how much “fresh water” is wasted when its mixed with salt water ? If everyone did that fresh water sources would soon dry up ?
One mega-watt from one square meter of membrane!?!? Is this a typo?