What is the purpose of the switch shown in this schematic diagram?

|
|
This device is known as a switch, and its purpose in this circuit is to establish or interrupt the electrical continuity of the circuit in order to control the light bulb.
Beginning students often find the terminology for switches confusing, because the words open and closed sound similar to the terminology used for doors, but do not mean quite the same thing when used in reference to a switch! In order to help avoid confusion, ask the students how they may think of these terms in a way that is consistent with their meaning in the context of an electrical switch.
One analogy to use for the switch’s function that makes sense with the schematic is a drawbridge: when the bridge is down (closed), cars may cross; when the bridge is up (open), cars cannot.
What difference will it make if the switch is located in either of these two alternate locations in the circuit?

|
|

|
|
The choice of switch locations shown in the two alternate diagrams makes no difference at all. In either case, the switch exerts the same control over the light bulb.
This is a difficult concept for some students to master. Make sure they all understand the nature of electrical current and the importance of continuity throughout the entire circuit. Perhaps the best way for students to master this concept is to actually build working battery-switch-lamp circuits. Remind them that their “research” of these worksheet questions is not limited to book reading. It is not only valid, but preferable for them to experiment on their own, so long as the voltages are low enough that no shock hazard exists.
One analogy to use for the switch’s function that makes sense with the schematic is a drawbridge: when the bridge is down (closed), cars may cross; when the bridge is up (open), cars cannot.
Does this switch (in the closed state) have a low resistance or a high resistance between its terminals?

|
|
A closed switch is supposed to have low resistance between its terminals.
Ask the students what it would mean if a closed switch actually measured having high resistance between its terminals. Knowing what the measurements of any electrical component ought to be is a very important skill for troubleshooting.
How might you use a meter (or a conductivity/continuity tester) to determine whether this electrical switch is in the open or closed state?

|
|
Most multimeters have a “resistance” measurement range (“Ohms scale”) that may be used to check continuity. Either using a meter or a conductivity/continuity tester, measure between the two screw terminals of this switch: if the resistance is low (good conductivity), then the switch is closed. If the measured resistance is infinite (no conductivity), then the switch is open.
This is another question which lends itself well to experimentation. A vitally important skill for students to develop is how to use their test equipment to diagnose the states of individual components.
An inexpensive source of simple (SPST) switches is a hardware store: use the same type of switch that is used in household light control. These switches are very inexpensive, rugged, and come with heavy-duty screw terminals for wire attachment. When used in small battery-powered projects, they are nearly indestructible!
Determine if the light bulb will de-energize for each of the following breaks in the circuit. Consider just one break at a time:

|
|
This question is an important one in the students’ process of learning troubleshooting. Emphasize the importance of inductive thinking: deriving general principles from specific instances. What does the behavior of this circuit tell us about electrical continuity?
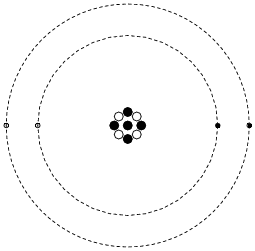
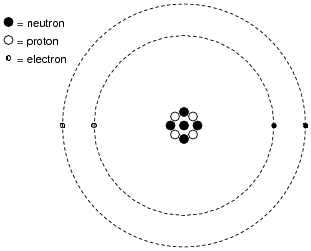
Shown here is a simplified representation of an atom: the smallest division of matter that may be isolated through physical or chemical methods.
|
|
Inside of each atom are several smaller bits of matter called particles. Identify the three different types of “elementary” particles inside an atom, their electrical properties, and their respective locations within the atom.
|
|
Neutrons reside in the center (“nucleus”) of the atom, as do protons. Neutrons are electrically neutral (no charge), while protons have a positive electrical charge. Electrons, which reside outside the nucleus, have negative electrical charges.
Most, if not all, students will be familiar with the “solar system” model of an atom, from primary and secondary science education. In reality, though, this model of atomic structure is not that accurate. As far as anyone knows, the actual physical layout of an atom is much, much weirder than this!
A question that might come up in discussion is the definition of “charge.” I’m not sure if it is possible to fundamentally define what “charge” is. Of course, we may discuss “positive” and “negative” charges in operational terms: that like charges repel and opposite charges attract. However, this does not really tell us what charge actually is. This philosophical quandary is common in science: to be able to describe what something is in terms of its behavior but not its identity or nature.
Different types of atoms are distinguished by different numbers of elementary particles within them. Determine the numbers of elementary particles within each of these types of atoms:
Hint: look up each of these elements on a periodic table.
Each atom of carbon is guaranteed to contain 6 protons. Unless the atom is electrically charged, it will contain 6 electrons as well to balance the charge of the protons. Most carbon atoms contain 6 neutrons, but some may contain more or less than 6.
Each atom of hydrogen is guaranteed to contain 1 proton. Unless the atom is electrically charged, it will contain 1 electron as well to balance the charge of the one proton. Most hydrogen atoms contain no neutrons, but some contain either one or two neutrons.
Each atom of helium is guaranteed to contain 2 protons. Unless the atom is electrically charged, it will contain 2 electrons as well to balance the charge of the protons. Most helium atoms contain 2 neutrons, but some may contain more or less than 2.
Each atom of aluminum is guaranteed to contain 13 protons. Unless the atom is electrically charged, it will contain 13 electrons as well to balance the charge of the protons. Most aluminum atoms contain 14 neutrons, but some may contain more or less than 14.
While you’re researching the numbers of particles inside each of these atom types, you may come across these terms: atomic number and atomic mass (sometimes called atomic weight). Be prepared to discuss what these two terms mean.
Be sure to ask your students what definitions they found for “atomic number” and “atomic mass”.
It is highly recommended that students seek out periodic tables to help them with their research on this question. The ordering of elements on a periodic table may provoke a few additional questions such as, “Why are the different elements arranged like this?” This may build to a very interesting discussion on basic chemistry, so be prepared to engage in such an interaction on these subjects if necessary.
Of the three types of “elementary particles” constituting atoms, determine which type(s) influence the following properties of an element:
It never ceases to fascinate me how many of the basic properties of elements is determined by a simple integer count of particles within each atom’s nucleus.
In the answer, I introduce the word isotope. Let students research what this term means. Don’t simply tell them!
The Greek word for amber (fossilized resin) is elektron. Explain how this came to be the word describing a certain type of subatomic particle (electron).
When a piece of amber is rubbed with a cloth, a static electric charge develops on both objects. Early experimenters postulated the existence of an invisible fluid that was transferred between the amber and the cloth. Later, it was discovered that tiny sub-atomic particles constituted this “fluid,” and the name electron was given to them.
This question provides a good opportunity to discuss the history of electricity, and how its understanding and mastery has dramatically changed peoples’ lives. Be sure to ask questions about Benjamin Franklin and the modeling of electricity as a fluid. Scientific discovery is often assisted by models, but may also be hindered by them as well. Franklin’s model of electricity as a fluid has done both (conventional versus electron flow notation)!
What does it mean for an object to have an electric charge? Give an example of an object receiving an electric charge, and describe how that charged object might behave.
For an object to be electrically charged, it must have either a surplus or a deficit of electrons among its atoms.
A common example of electrically charging objects is rubbing latex balloons against wool clothing, or brushing your hair with a plastic comb. The consequences of these electric charges are very easy to perceive!
This question naturally leads to a discussion on atomic theory. Encourage your students to discuss and explore simple models of the atom, and how they serve to explain electricity in terms of electron placement and motion.
How many electrons are contained in one coulomb of charge?
There are 6.25 ×1018 electrons in one coulomb of charge. What would this appear as without the use of scientific notation? Write this same figure using the most appropriate metric prefix.
A little math review here: using scientific notation to denote very large (or very small) numbers.
What is happening when two objects are rubbed together and static electricity results?
When certain combinations of materials are rubbed together, the rubbing action transfer electrons from the atoms of one material to the atoms of the other. This imbalance of electrons leaves the former material with a positive charge and the latter with a negative charge.
The terms “positive” and “negative” seem backward in relation to the modern concept of electrons as charge carriers. Be sure to discuss the historical aspect of this terminology (Benjamin Franklin’s conjecture), and the subsequent designation of an electron’s individual charge as “negative.”
It is much easier to electrically “charge” an atom than it is to alter its chemical identity (say, from lead into gold). What does this fact indicate about the relative mobility of the elementary particles within an atom?
Electrons are much easier to remove from or add to an atom than protons are. The reason for this is also the solution to the paradox of why protons bind together tightly in the nucleus of an atom despite their identical electrical charges.
Discuss with your students the importance of this fact: that electrons may be added to or taken from an atom rather easily, but that protons (and neutrons for that matter) are very tightly “bound” within an atom. What might atoms behave like if their protons were not so tightly bound as they are?
We know what happens to the electrons of some atoms when substances are rubbed together. What might happen to those substances if protons were not as tightly bound together as they are?
Explain what the electrical terms voltage, current, and resistance mean, using your own words.
Voltage: electrical “pressure” between two different points or locations.
Current: the flow of electrons.
Resistance: opposition, or “friction,” to the flow of electrons.
Voltage, current and resistance is related through Ohm’s Law.
While it is easy enough for students to look up definitions for these words from any number of references, it is important that they be able to cast them into their own words. Remembering a definition is not the same as really understanding it, and if a student is unable to describe the meaning of a term using their own words then they definitely do not understand it! It is also helpful to encourage students to give real-life examples of these terms.
Describe what “electricity” is, in your own words.
If you’re having difficulty formulating a definition for “electricity,” a simple definition of “electric current” will suffice. What I’m looking for here is a description of how an electric current may exist within a solid material such as a metal wire.
This question is not as easy to answer as it may first appear. Certainly, electric current is defined as the “flow” of electrons, but how do electrons “flow” through a solid material such as copper? How does anything flow through a solid material, for that matter?
Many scientific disciplines challenge our “common sense” ideas of reality, including the seemingly solid nature of certain substances. One of the liberating aspects of scientific investigation is that it frees us from the limitations of direct sense perception. Through structured experimentation and rigorous thinking, we are able to “see” things that might otherwise be impossible to see. We certainly cannot see electrons with our eyes, but we can detect their presence with special equipment, measure their motion by inference from other effects, and prove empirically that they do in fact exist.
In this regard, scientific method is a tool for the expansion of human ability. Your students will begin to experience the thrill of “working with the invisible” as they explore electricity and electric circuits. It is your task as an instructor to foster and encourage this sense of wonder in your students’ work.
What is the difference between materials classified as conductors versus those classified as insulators, in the electrical sense of these words?
Electrical “conductors” offer easy passage of electric current through them, while electrical “insulators” do not. The fundamental difference between an electrical “conductor” and an electrical “insulator” is how readily electrons may drift away from their respective atoms.
For an illustration of electron mobility within a metallic substance, research the terms electron gas and “Sea of electrons” in a chemistry reference book.
It is important to realize that electrical “conductors” and “insulators” are not the same as thermal “conductors” and “insulators.” Materials that are insulators in the electrical sense may be fair conductors of heat (certain silicone gels used as heat-transfer fluids for heat sinks, for instance). Materials that are conductors in the electrical sense may be fair insulators in the thermal sense (conductive plastics, for example).
Identify several substances that are good conductors of electricity, and several substances that are good insulators of electricity.
It is very easy to research (and test!) whether or not various substances are either conductors or insulators of electricity. I leave this task in your very capable hands.
If students have access to simple multimeters, they may perform conductivity tests on various substances with them. This is a fun and interesting classroom activity!
In the simplest terms you can think of, define what an electrical circuit is.
An electrical circuit is any continuous path for electrons to flow away from a source of electrical potential (voltage) and back again.
Although definitions are easy enough to research and repeat, it is important that students learn to cast these concepts into their own words. Asking students to give practical examples of “circuits” and “non-circuits” is one way to ensure deeper investigation of the concepts than mere term memorization.
The word “circuit,” in vernacular usage, often refers to anything electrical. Of course, this is not true in the technical sense of the term. Students will come to realize that many terms they learn and use in an electricity or electronics course are actually mis-used in common speech. The word “short” is another example: technically it refers to a specific type of circuit fault. Commonly, though, people use it to refer to any type of electrical problem.
What is the difference between DC and AC electricity? Identify some common sources of each type of electricity.
DC is an acronym meaning Direct Current: that is, electrical current that moves in one direction only. AC is an acronym meaning Alternating Current: that is, electrical current that periodically reverses direction (“alternates”).
Electrochemical batteries generate DC, as do solar cells. Microphones generate AC when sensing sound waves (vibrations of air molecules). There are many, many other sources of DC and AC electricity than what I have mentioned here!
Discuss a bit of the history of AC versus DC in early power systems. In the early days of electric power in the United States of America, there was a heated debate between the use of DC versus AC. Thomas Edison championed DC, while George Westinghouse and Nikola Tesla advocated AC.
It might be worthwhile to mention that almost all the electric power in the world is generated and distributed as AC (Alternating Current), and not as DC (in other words, Thomas Edison lost the AC/DC battle!). Depending on the level of the class you are teaching, this may or may not be a good time to explain why most power systems use AC. Either way, your students will probably ask why, so you should be prepared to address this question in some way (or have them report any findings of their own!).
Suppose you are building a cabin far away from electric power service, but you desire to have electricity available to energize light bulbs, a radio, a computer, and other useful devices. Determine at least three different ways you could generate electrical power to supply the electric power needs at this cabin.
There are several different devices capable of producing electrical power for this cabin of yours:
For each of these devices, what is its operating principle, and where does it obtain its energy from?
For each of these electric power “sources,” there is a more fundamental source of energy. People often mistakenly think of generator devices as magic sources of energy, where they are really nothing more than energy converters: transforming energy from one form to another.
Where does the electricity come from that powers your home, or your school, or the streetlights along roads, or the many business establishments in your city? You will find that there are many different sources and types of sources of electrical power. In each case, try to determine where the ultimate source of that energy is.
For example, in a hydroelectric dam, the electricity is generated when falling water spins a turbine, which turns an electromechanical generator. But what continually drives the water to its “uphill” location so that the process is continuous? What is the ultimate source of energy that is being harnessed by the dam?
Some sources of electrical power:
A great point of conversation here is that almost all “sources” of energy have a common origin. The different “sources” are merely variant expressions of the same true source (with exceptions, of course!).
Given a battery and a light bulb, show how you would connect these two devices together with wire so as to energize the light bulb:

|
|
This is the simplest option, but not the only one.

|
|
This question gives students a good opportunity to discuss the basic concept of a circuit. It is very easy to build, safe, and should be assembled by each student individually in class. Also, emphasize how simple circuits like this may be assembled at home as part of the “research” portion of the worksheet. To research answers for worksheet questions does not necessarily mean the information has to come from a book! Encourage experimentation when the conditions are known to be safe.
Have students brainstorm all the important concepts learned in making this simple circuit. What general principles may be derived from this particular exercise?
Draw an electrical schematic diagram of a circuit where a battery provides electrical energy to a light bulb.
This schematic diagram is not the only valid way to show a battery powering a light bulb:

|
|
Other orientations of the components within the diagram are permissible. What matters, though, is for there to be a single, continuous path for electric current from the battery, to the light bulb, and back to the other terminal of the battery.
Impress upon the students the importance of learning to “communicate” in the language of schematic diagrams. The symbols and conventions learned here are international, and not limited to use in the United States.
Most electrical wire is covered in a rubber or plastic coating called insulation. What is the purpose of having this “insulation” covering the metal wire?
The purpose of insulation covering the metal part of an electrical wire is to prevent accidental contact with other conductors of electricity, which might result in an unintentional electric current through those other conductors.
Not only is this question practical from the standpoint of understanding circuit function, but also from the perspective of electrical safety. Why is it important for wires to be insulated? Are overhead power lines insulated like the wires used in classroom projects? Why or why not? How were electrical wires insulated before the advent of modern plastics technology?
In the early days of electrical wiring, wires used to be insulated with cotton. This is no longer accepted practice. Explain why.
Cotton, like many natural fibers, is an electrical insulator . . . until it becomes wet!
This question affords the opportunity to discuss electrical safety with regard to clothing (often made of cotton). Does dry clothing offer insulation to electricity like the old-style cotton wire insulation? Can cotton clothing be trusted to insulate you safely from hazardous voltage?
How could a battery, a light bulb, and some lengths of metal wire be used as a conductivity tester, to test the ability of different objects to conduct electricity?
The following circuit would function as a simple continuity tester. Simply place the open wire ends in contact with the object to be tested, and the light bulb will indicate whether or not the object conducts electricity to any substantial degree:

|
|
Not only is this question an opportunity to solve a problem, but it lends itself well to simple and safe experimentation. Encourage students to build their own conductivity testers and test various substances with them.
Suppose we had a long length of electrical cable (flexible tubing containing multiple wires) that we suspected had some broken wires in it. Design a simple testing circuit that could be used to check each of the cable’s wires individually.

|
|

|
|
A significant portion of electrical/electronic circuit problems are caused by nothing more complex than broken wire connections, or faults along the length of wires. Testing cables for wire breaks is a very practical exercise.
The same technique may be used to “map” wires from one end of a cable to the other, in the event that the wires are not color-coded or otherwise made identifiable.
How long will it take for the light bulb to receive electrical power once the battery is connected to the rest of the circuit?

|
|
Approximately 11 milliseconds (0.0107 seconds).
Electricity is fast: the effects of electron motion travel at approximately the speed of light (186,000 miles per second). Actual average electron velocity, on the other hand, is very, very slow. A convenient analogy I’ve used to illustrate how electrons may move slowly yet have rapid effect is that of a closed-loop hydraulic system. When the valve is opened, fluid motion throughout the system is immediate (actually, the motion progresses at the speed of sound through the fluid - very fast!), yet the actual velocity of fluid motion is much slower.
Incidentally, the double-chevron symbols indicate an electrical connector pair (plug and jack; male and female).
A 22-gauge metal wire three feet in length contains approximately 28.96 ×1021 “free” electrons within its volume. Suppose this wire is placed in an electric circuit conducting a current equal to 6.25 ×1018 electrons per second. That is, if you were able to choose a spot along the length of this wire and were able to count electrons as they drifted by that spot, you would tally 6,250,000,000,000,000,000 electrons passing by each second. (This is a reasonable rate for electric current in a wire of this size.)
Calculate the average velocity of electrons through this wire.
Average electron velocity = 0.000647 feet per second, or 6.47 ×10−4 ft/s. This is very slow: only 0.00777 inches per second, or 0.197 millimeters per second!
Despite the rapid progression of the effects of electron motion throughout a circuit (i.e. approximately the speed of light), the actual electron velocity is extremely slow by comparison.
Base figures used in this calculation are as follows:
Questions like this may be challenging to students without a strong math or science background. One problem-solving strategy I have found very useful is to simplify the terms of a problem until a solution becomes obvious, then use that simplified example to establish a pattern (equation) for obtaining a solution given any initial parameters. For instance, what would be the average electron velocity if the current were 28.96 ×1021 electrons per second, the same figure as the number of free electrons residing in the wire? Obviously, the flow velocity would be one wire length per second, or 3 feet per second. Now, alter the current rate so that it is something closer to the one given in the problem (6.25 ×1018), but yet still simple enough to calculate mentally. Say, half the first rate: 14.48 ×1021 electrons per second. Obviously, with a flow rate half as much, the velocity will be half as well: 1.5 feet per second instead of 3 feet per second. A few iterations of this technique should reveal a pattern for solution:
|
Where,
v = Average electron velocity (feet per second)
I = Electric current (electrons per second)
Q = Number of electrons contained in wire
It is also very helpful to have knowledgeable students demonstrate their solution techniques in front of the class so that others may learn novel methods of problem-solving.
What do the symbols with the question marks next to them refer to? In the circuit shown, would the light bulb be energized?

|
|
These are ground symbols, and they can either refer to connections made to a common conductor (such as the metal chassis of an automobile or circuit enclosure), or the actual earth (usually via metal rods driven into the dirt).
Ask the students about the relative conductivities of metal chassis versus dirt (earth ground). Is a current pathway formed by two metal chassis grounds equivalent to a current pathway formed by two earth grounds? Why or why not? What conditions may affect these relative conductivities?
Shown here is a simplified representation of an electrical power plant and a house, with the source of electricity shown as a battery, and the only electrical “load” in the house being a single light bulb:

|
|
Why would anyone use two wires to conduct electricity from a power plant to a house, as shown, when they could simply use one wire and a pair of ground connections, like this?

|
|
This is not a practical solution, even though it would only require half the number of wires to distribute electrical power from the power plant to each house! The reason this is not practical is because the earth (dirt) is not a good enough conductor of electricity. Wires made of metal conduct electricity far more efficiently, which results in more electrical power delivered to the end user.
Discuss the fact that although the earth (dirt) is a poor conductor of electricity, it may still be able to conduct levels of current lethal to the human body! The amount of current necessary to light up a household light bulb is typically far in excess of values lethal for the human body.
What, exactly, is a short circuit? What does it mean if a circuit becomes shorted? How does this differ from an open circuit?
A short circuit is a circuit having very little resistance, permitting large amounts of current. If a circuit becomes shorted, it means that a path for current formerly possessing substantial resistance has been bypassed by a path having negligible (almost zero) resistance.
Conversely, an open circuit is one where there is a break preventing any current from going through at all.
Discuss with your students some of the potential hazards of short circuits. It will then be apparent why a “short circuit” is a bad thing. Ask students if they can think of any realistic circumstance that could lead to a short-circuit developing.
I have noticed over several years of teaching electronics that the terms “short” or “short-circuit” are often used by new students as generic labels for any type of circuit fault, rather than the specific condition just described. This is a habit that must be corrected, if students are to communicate intelligently with others in the profession. To say that a component “is shorted” means a very definite thing: it is not a generic term for any type of circuit fault.
What would have to happen in this circuit for it to become shorted? In other words, determine how to make a short circuit using the components shown here:

|
|

|
|
In real life, of course, short circuits are usually things to be avoided. Discuss with your students why short circuits are generally undesirable, and what role wire insulation plays in preventing them.
When lightning strikes, nearby magnetic compass needles may be seen to jerk in response to the electrical discharge. No compass needle deflection results during the accumulation of electrostatic charge preceding the lightning bolt, but only when the bolt actually strikes. What does this phenomenon indicate about voltage, current, and magnetism?
The presence of an electric current will produce a magnetic field, but the mere presence of a voltage will not. For more detail on the historical background of this scientific discovery, research the work of Hans Christian Oersted in the early 1820’s.
The discovery of electromagnetism was nothing short of revolutionary in Oersted’s time. It paved the way for the development of electric motors, among other useful electrical devices.
Just as electricity may be harnessed to produce magnetism, magnetism may also be harnessed to produce electricity. The latter process is known as electromagnetic induction. Design a simple experiment to explore the phenomenon of electromagnetic induction.
Perhaps the easiest way to demonstrate electromagnetic induction is to build a simple circuit formed from a coil of wire and a sensitive electrical meter (a digital meter is preferred for this experiment), then move a magnet past the wire coil. You should notice a direct correlation between the position of the magnet relative to the coil over time, and the amount of voltage or current indicated by the meter.
Many students improperly assume that electromagnetic induction may take place in the presence of static magnetic fields. This is not true. The simple experimental setup described in the “Answer” section for this question is sufficient to dispel that myth, and to illuminate students’ understanding of this principle. Incidentally, this activity is a great way to get students started thinking in calculus terms: relating one variable to the rate of change over time of another variable.
A large audio speaker may serve to demonstrate both the principles of electromagnetism and of electromagnetic induction. Explain how this may be done.
I won’t tell you how to set up or do the experiment, but I will show you an illustration of a typical audio speaker:

|
|
The “voice coil” is attached to the flexible speaker cone, and is free to move along the long axis of the magnet. The magnet is stationary, being solidly anchored to the metal frame of the speaker, and is centered in the middle of the voice coil.
This experiment is most impressive when a physically large (i.e. “woofer”) speaker is used.
Follow-up question: identify some possible points of failure in a speaker which would prevent it from operating properly.
Since not everyone has ready access to a large speaker for this kind of experiment, it may help to have one or two “woofer” speakers located in the classroom for students to experiment with during this phase of the discussion. Any time you can encourage students to set up impromptu experiments in class for the purpose of exploring fundamental principles, it is a Good Thing.
What do you think might happen if someone were to gently tap on the cone of one of these speakers? What would the other speaker do? In terms of electromagnetism and electromagnetic induction, explain what is happening.

|
|
Try this experiment yourself, using a long pair of wires to separate the two speakers from each other by a significant distance. Listen and feel the speaker on your end while someone else taps on the other speaker, then trade roles.
Not only does this experiment illustrate the dual principles of electromagnetism and electromagnetic induction, but it also demonstrates how easy it is to set up a simple sound-powered audio telephony system.
It is highly recommended to have an identical pair of “woofer” speakers located in the classroom for this experiment, as well as a long length of twin-wire cable (an old piece of extension cord wire works well for this purpose, with alligator-clip “jumper” wires to make the connections).
Suppose someone mechanically couples an electric motor to an electric generator, then electrically couples the two devices together in an effort to make a perpetual-motion machine:

|
|
Why won’t this assembly spin forever, once started?
This will not work because neither the motor nor the generator is 100% efficient.
The easy answer to this question is “the Law of Conservation of Energy (or the Second Law of Thermodynamics) forbids it,” but citing such a “Law” really doesn’t explain why perpetual motion machines are doomed to failure. It is important for students to realize that reality is not bound to the physical “Laws” scientists set; rather, what we call “Laws” are actually just descriptions of regularities seen in nature. It is important to emphasize critical thinking in a question like this, for it is no more intellectually mature to deny the possibility of an event based on dogmatic adherence to a Law than it is to naively believe that anything is possible.
Published under the terms and conditions of the Creative Commons Attribution License

In Partnership with Renesas Electronics

by Robert Keim

by Jake Hertz

